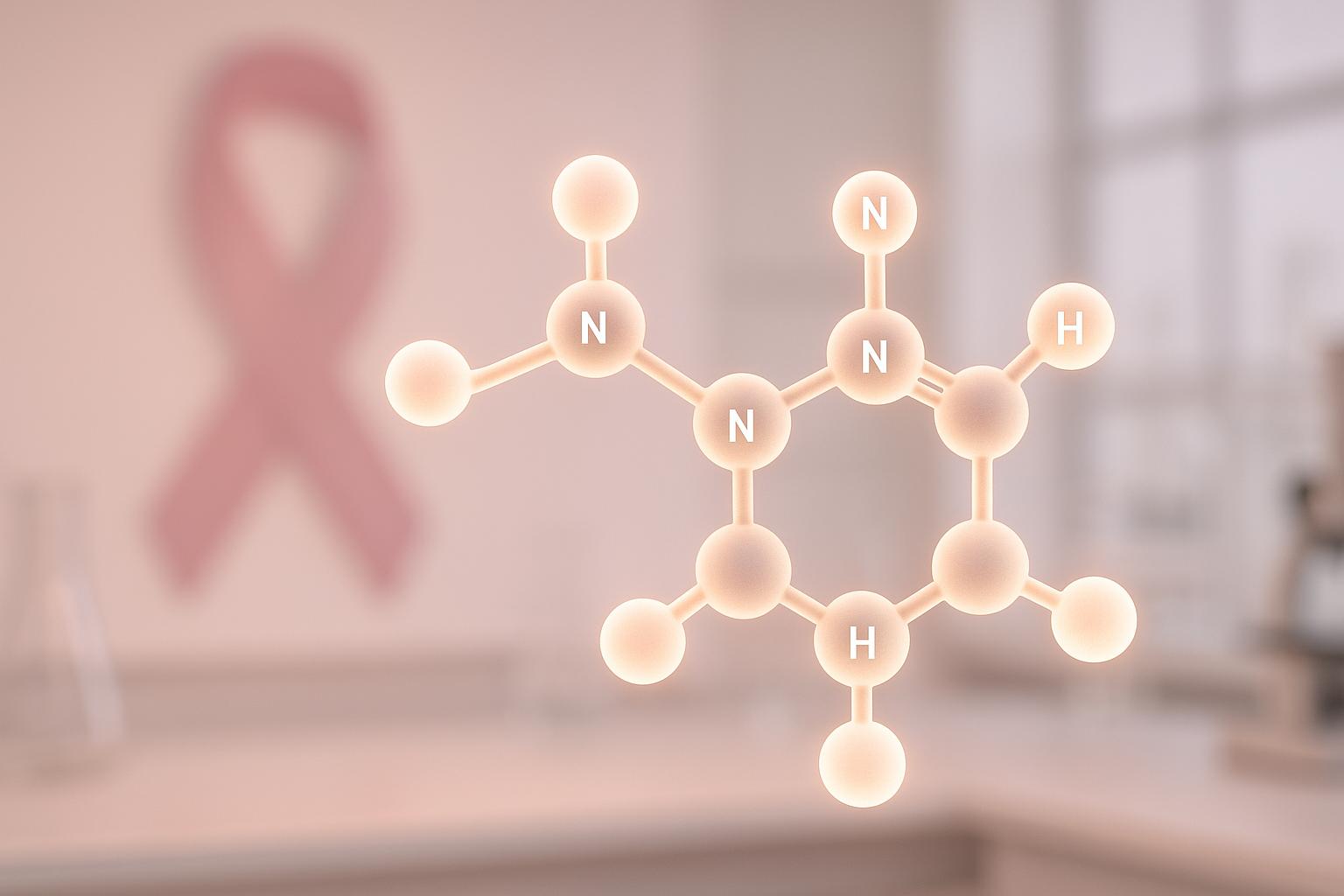
Metformin, a diabetes medication approved in 1995, is gaining attention for its potential in reducing breast cancer risk. Research highlights five key ways it may work against cancer:
- AMPK/mTOR Pathway Inhibition: Metformin activates AMPK, which suppresses mTOR, slowing cancer cell growth.
- Lower Insulin and IGF-1 Levels: It reduces insulin and IGF-1, hormones linked to tumor growth.
- Metabolic Disruption: The drug disrupts cancer cells' energy supply by targeting how they produce energy.
- Cell Cycle Arrest and Apoptosis: Metformin halts cancer cell division and triggers their death.
- Immune System Effects: It reduces inflammation and enhances the immune system’s ability to fight cancer.
These mechanisms show promise, especially for individuals with a higher risk of breast cancer or those with Type 2 diabetes. While more research is needed, metformin’s multi-faceted approach could make it a valuable tool in cancer prevention and treatment strategies.
Oxford University surgical lectures: Effect of metformin on breast cancer metabolism
1. AMPK/mTOR Pathway Inhibition
Metformin works by activating the AMPK pathway, which acts as a cellular energy sensor. This activation helps suppress processes like protein synthesis, fatty acid production, and cholesterol production, all of which are critical for tumor growth.
When AMPK is activated, it inhibits mTOR, a key regulator of cell growth. This is particularly important in breast cancers with PIK3CA mutations or PTEN loss, where mTOR activity is often unchecked. AMPK achieves this inhibition by interacting with the TSC2 complex and directly targeting RAPTOR, effectively slowing down cancer cell proliferation.
Evidence supports this mechanism. A retrospective study found that diabetic breast cancer patients taking metformin had a 24% complete response rate, compared to just 8% in those not using it. Animal studies further back this up - treatment with 75 μM metformin for 24 hours increased AMPK activity, suppressed mTOR, and reduced cancer cell survival.
Beyond this, metformin's action on the AMPK/mTOR pathway also targets cancer stem cells. This is crucial, as it can lower the chances of treatment resistance and recurrence.
Up next, we’ll look at metformin’s effect on insulin and IGF-1 signaling.
2. Lower Insulin and IGF-1 Signaling
Metformin plays a key role in reducing circulating insulin and IGF-1 levels, both of which are known to encourage cancer cell growth. This reduction is a critical step in slowing tumor progression.
Here's how it works: metformin decreases glucose production in the liver and improves glucose uptake in muscles. This leads to lower insulin levels, which is important because insulin doesn’t just manage blood sugar - it can also directly promote tumor growth in epithelial tissues or indirectly impact cancer development through factors like insulin-like growth factors (IGFs), sex hormones, and adipokines.
Metformin also lowers circulating IGF-1 levels. A study showed that patients taking metformin had significantly lower IGF-1 levels compared to those in the control group. Interestingly, patients whose IGF-1 levels rose after treatment were more likely to experience disease progression. This mechanism is particularly relevant for individuals with insulin resistance, as we'll discuss further.
Why This Matters for Diabetic Patients
For diabetic patients, reducing insulin and IGF-1 levels isn’t just about controlling blood sugar - it’s also about lowering cancer risk. Studies show that type 2 diabetes increases the risk of breast cancer by 23% and raises cancer-related mortality by 38%.
One study by Campagnoli et al. found that metformin use in women with breast cancer reduced insulin and testosterone levels, which helped alleviate insulin resistance. In fact, research suggests that type 2 diabetic patients taking metformin have a 30% lower chance of developing cancer compared to those using other medications.
Next, we’ll look at how metformin disrupts cancer’s energy supply by triggering metabolic changes.
3. Metabolic Changes and Warburg Effect Disruption
Focusing on how cancer cells produce energy is a key strategy in breast cancer prevention. Unlike healthy cells, cancer cells often depend on a process called the Warburg effect. This involves a shift from oxidative phosphorylation to aerobic glycolysis, even when oxygen is plentiful. This altered energy production supports tumor growth and creates a highly glycolytic environment. Additionally, the buildup of lactic acid from this process acidifies the tumor's surroundings, which can promote tumor progression. Metformin takes advantage of these metabolic changes to cut off the energy supply cancer cells rely on.
How Metformin Interrupts This Process
Metformin exploits this metabolic weakness by targeting complex I of the mitochondrial electron-transport chain. By doing so, it increases the intracellular AMP/ADP ratio, which puts the cells under energetic stress. This stress reduces oxygen consumption and hampers the TCA cycle, leaving cancer cells unable to sustain their preferred energy production pathway.
Starving Cancer Cells of Glucose
Metformin also works by cutting off the glucose supply cancer cells depend on. The drug can accumulate in the mitochondrial matrix at concentrations up to 1,000 times higher than in the surrounding environment. By binding to the HK2 pocket, metformin blocks the enzyme's ability to process glucose, effectively starving the cancer cells. Furthermore, it reduces the expression of LDHA, a key enzyme in glycolysis, which further disrupts the Warburg effect and slows tumor growth.
Evidence from Research
Research backs up metformin's ability to disrupt cancer metabolism. For instance, in studies on skin squamous cell carcinoma, metformin reduced aerobic glycolysis while boosting oxidative phosphorylation. When combined with photodynamic therapy, it also increased the generation of reactive oxygen species, enhancing its anti-cancer effects.
Making Cancer Cells More Vulnerable
By reversing the Warburg effect, metformin doesn’t just disrupt energy production - it also makes cancer cells more susceptible to other treatments. This metabolic shift can enhance the effectiveness of therapies by triggering apoptosis and improving overall treatment outcomes.
sbb-itb-cad6158
4. Cell Cycle Arrest and Programmed Cell Death
Metformin doesn't just disrupt cancer cell metabolism - it actively halts their division and prompts them to self-destruct. Acting like a control mechanism, it stops breast cancer cells from multiplying and triggers programmed cell death (apoptosis). These actions work alongside the drug's metabolic effects to combat cancer.
Stopping Cancer Cells from Dividing
Metformin is particularly effective at causing cell cycle arrest during the G0/G1 phase. Studies reveal that it increases the number of cells in this arrested state by over 10% compared to untreated cells. This process involves several molecular changes: metformin reduces cyclin D1 levels while boosting the activity of CDK inhibitors p27Kip1 and p21Cip1. As researcher Yongxian Zhuang explained:
"Cell cycle arrest in response to metformin requires CDK inhibitors in addition to AMPK activation and cyclin D1 downregulation."
Triggering Programmed Cell Death
When halting cell division isn't enough, metformin goes a step further by initiating apoptosis. It achieves this through a mitochondria-driven pathway, increasing reactive oxygen species (ROS) that disrupt the mitochondrial membrane potential. This disruption tilts the balance of life-and-death proteins, leading to higher levels of the pro-apoptotic protein BAX and lower levels of anti-apoptotic proteins like BCL-2 and MCL-1.
Laboratory Evidence
The apoptotic effects of metformin are well-documented in lab studies. In MDA-MB-231 breast cancer cells, apoptosis rose from 7.0% with 5 mM metformin to 39.6% with 20 mM metformin. Even in the less sensitive MDA-MB-435 cell line, apoptosis increased from 2.9% to 17.7%. Colony formation assays further highlight these effects: at 20 mM, colony formation in MCF7 and 4T1 cells dropped by over 60%, and at 50 mM, it was completely suppressed.
Selective Targeting of Cancer Cells
One of metformin's most promising traits is its selective toxicity - it primarily targets cancer cells while sparing normal ones. This precision is particularly crucial for eradicating cancer stem cells, which are often resistant to conventional treatments. In MCF-7 breast cancer cells treated with 10 mM metformin over 24, 48, and 72 hours, researchers observed G0/G1 cell cycle arrest, increased apoptosis, and necrosis linked to oxidative stress. These effects were accompanied by reduced activity in survival pathways (IRβ, Akt, and ERK1/2) and increased expression of death-promoting factors like p-AMPK, FOXO3a, p27, Bax, and cleaved caspase-3.
Enhancing the Effectiveness of Cancer Therapies
Metformin's ability to arrest the cell cycle and induce apoptosis may also amplify the effectiveness of other cancer treatments. By weakening cancer cells, it can make them more susceptible to therapies such as radiotherapy, chemotherapy, and immunotherapy. As Queiroz et al. observed:
"Our study further reinforces the potential benefit of metformin in cancer treatment and provides novel mechanistic insight into its antiproliferative role."
These combined mechanisms of cell cycle arrest and apoptosis highlight metformin's multifaceted approach to combating breast cancer, paving the way for further exploration of its therapeutic potential.
5. Anti-inflammatory and Immune System Effects
Metformin does more than just stop cell division; it also disrupts tumor growth by reshaping the immune system. By influencing immune responses, metformin creates conditions that make it harder for breast cancer to thrive. Here's how it works:
Reducing Chronic Inflammation
Chronic inflammation is a known contributor to breast cancer risk, and metformin helps tackle this issue by activating AMPK and suppressing NF-κB signaling. This is especially critical in individuals with obesity, where excess fat tissue leads to persistent, low-grade inflammation driven by macrophages. Research shows that metformin lowers key pro-inflammatory markers like IL-6 and TNF-α in both lab models and animal studies.
Shifting Immune Cell Balance
Metformin doesn't just reduce inflammation - it also changes the behavior of immune cells. It encourages macrophages to adopt an anti-inflammatory M2 phenotype, even in conditions that would normally favor the pro-inflammatory M1 type. This shift reduces aromatase-positive macrophages, which are linked to higher breast cancer risk.
Enhancing Cancer Surveillance
Another way metformin supports the immune system is by improving its ability to detect and attack cancer cells. Studies have found that it strengthens the antitumor activity of natural killer T (NKT) cells and adjusts T cell behavior. For example, it increases apoptotic markers like FasL while decreasing immunosuppressive markers such as PD-1 and KLRG1. Similarly, natural killer (NK) cells show heightened activation, with increased levels of FasL, NKp46, and IFN-γ, making them more effective at targeting cancer cells.
Improving Treatment Outcomes
Metformin's immune-regulating effects also enhance the effectiveness of cancer treatments. Clinical studies reveal that patients using metformin often have better tumor immune profiles and improved treatment responses. In one study involving 40 patients receiving preoperative systemic therapy, those on metformin had a higher rate of pathological complete response. They also showed favorable changes in tumor-infiltrating immune cells, including fewer M2-type macrophages and increased levels of CD3(+) and CD8(+) lymphocytes.
Comprehensive Immune Remodeling
In the 4T1 breast cancer model, metformin delayed tumor growth while boosting immune markers tied to tumor-killing activity. It increased IFN-γ⁺ NKT cells and cytotoxic markers like CD107a, while reducing immunosuppressive factors such as PD-1, FoxP3, and IL-10 in spleen cells. Within tumors, these changes included higher levels of beneficial immune markers and fewer regulatory and suppressor cells, creating an environment that supports tumor destruction.
Together, these immune-related effects make it much harder for breast cancer to develop and progress. By reshaping the immune system, metformin helps create an inhospitable environment for cancer.
Mechanism Comparison Table
The table below summarizes five key mechanisms, outlining how each pathway influences breast cancer development and treatment.
| Mechanism | Biological Pathway | Impact on Breast Cancer | Supporting Evidence | Subtype Relevance |
|---|---|---|---|---|
| AMPK/mTOR Pathway Inhibition | Mitochondrial complex I inhibition activates AMPK, which suppresses mTOR. | Reduces energy production and protein synthesis in cancer cells. | Diabetic breast cancer patients had a 24% complete response rate compared to 8% without metformin. | More effective under low-glucose conditions; glucose modulation has minimal impact on triple-negative cells. |
| Lower Insulin/IGF-1 Signaling | Reduced gluconeogenesis lowers insulin/IGF-1 levels, suppressing PI3K/AKT signaling. | Limits growth signals that promote cancer development. | The Sister Study found a 38% risk reduction with ≥10 years of use (HR 0.62). | Strongest effects seen in ER-positive breast cancer, especially with long-term use. |
| Metabolic Disruption | Disrupts the Warburg effect, forcing cancer cells to use less efficient energy pathways. | Forces cancer cells into less efficient energy production. | Glucose availability influences anti-proliferative effects. | Triple-negative cells show resistance to these effects under high-glucose conditions. |
| Cell Cycle Arrest | Halts cell division and triggers apoptosis. | Stops cancer cell division and induces programmed cell death. | Neoadjuvant studies reported decreased insulin receptor expression. | Effective across subtypes, but relies on achieving adequate drug concentrations. |
| Immune System Enhancement | Alters the tumor immune microenvironment by modifying macrophage polarization. | Creates a less favorable environment for cancer growth. | The METTEN trial showed a higher pathological complete response rate in the metformin arm (65.5% vs. 58.6%). | HER2-positive patients benefit most, with a 47% reduction in mortality (HR 0.53). |
Metformin's effectiveness is influenced by glucose levels and its concentration in the tumor microenvironment. For instance, in the NCIC MA.32 study involving 3,649 non-diabetic women, HER2-positive patients experienced a 36% reduction in disease recurrence (HR 0.64) and a 47% reduction in overall mortality (HR 0.53). This suggests HER2-positive tumors may be particularly sensitive to metformin's multi-faceted approach.
For ER-positive breast cancer, the insulin-lowering mechanism shows strong potential, especially with prolonged use. However, some studies have observed an increased risk of ER-negative cancers, highlighting the complex interplay between metformin and different breast cancer subtypes.
Additionally, the expression of membrane transporters like organic cation transporters (OCTs) in tumors plays a crucial role in metformin uptake. Higher OCT expression has been linked to better drug accumulation and stronger antitumor responses.
These mechanisms collectively provide a multi-layered defense against breast cancer, with varying impacts based on cancer subtype and individual patient factors. This underscores the diverse ways metformin can influence breast cancer treatment and outcomes.
Conclusion
Metformin's five mechanisms in breast cancer prevention offer a promising way to tackle both metabolic issues and breast cancer risks in the United States. These mechanisms work on multiple levels - ranging from AMPK/mTOR pathway inhibition to influencing inflammatory and immune responses - making them especially relevant for populations at higher risk.
The clinical takeaways are compelling. With the heightened risks tied to diabetes and cancer coexisting, metformin's dual role in addressing metabolic dysfunction and cancer pathways positions it as a valuable therapeutic option.
Research also points to subtype-specific effects, indicating that metformin's impact may vary depending on the breast cancer subtype, as previously discussed. This underscores the importance of moving toward personalized prevention strategies instead of relying on a universal approach.
Translating these findings into clinical practice isn't without challenges. Future studies should explore combining metformin with immunotherapy and investigate alternative delivery methods to ensure the drug reaches optimal tissue concentrations. For breast cancer patients with Type 2 diabetes, maintaining effective blood sugar control is crucial to enhancing metformin's cancer-fighting properties. By addressing both metabolic and cancer-related health concerns, this integrated approach paves the way for more effective prevention strategies.
FAQs
How does metformin target breast cancer cells through the AMPK/mTOR pathway while sparing normal cells?
Metformin works by activating AMPK, an important regulator of cellular energy. Once activated, AMPK suppresses the mTOR pathway, which plays a central role in protein synthesis and cell growth. This suppression is particularly effective against breast cancer cells, as they rely heavily on the mTOR pathway for rapid growth and division.
What makes metformin even more intriguing is its selective nature. Normal cells, which don't depend as much on the hyperactive mTOR pathway for survival, are less affected. This means metformin can target cancer cells while sparing healthy tissues, reducing potential harm and focusing its effects on disrupting cancer growth.
How does metformin enhance the immune system's ability to combat breast cancer?
Metformin plays a pivotal role in boosting the immune system's ability to combat breast cancer. It does this by enhancing the activity of IFN-γ+ NKT cells, which are crucial in identifying and destroying tumor cells. Additionally, it improves the overall function of immune cells, helping the body better recognize and fight cancer.
This combination of effects positions metformin as a potential ally in strengthening the body's natural defenses against breast cancer.
Which types of breast cancer respond best to metformin, and why?
Certain types of breast cancer, like HER2-positive and triple-negative breast cancers, show a stronger response to treatment with metformin. For HER2-positive cancers, metformin works by blocking HER2 tyrosine kinase activity, a key driver of tumor growth. On the other hand, triple-negative breast cancers - lacking hormone receptors and HER2 - are especially vulnerable to metformin’s effects, such as inducing apoptosis (programmed cell death) and interfering with cancer cell metabolism.
These variations in how the subtypes respond are tied to their distinct molecular pathways and metabolic characteristics, highlighting metformin’s potential as a targeted treatment for these cases.
.png)



