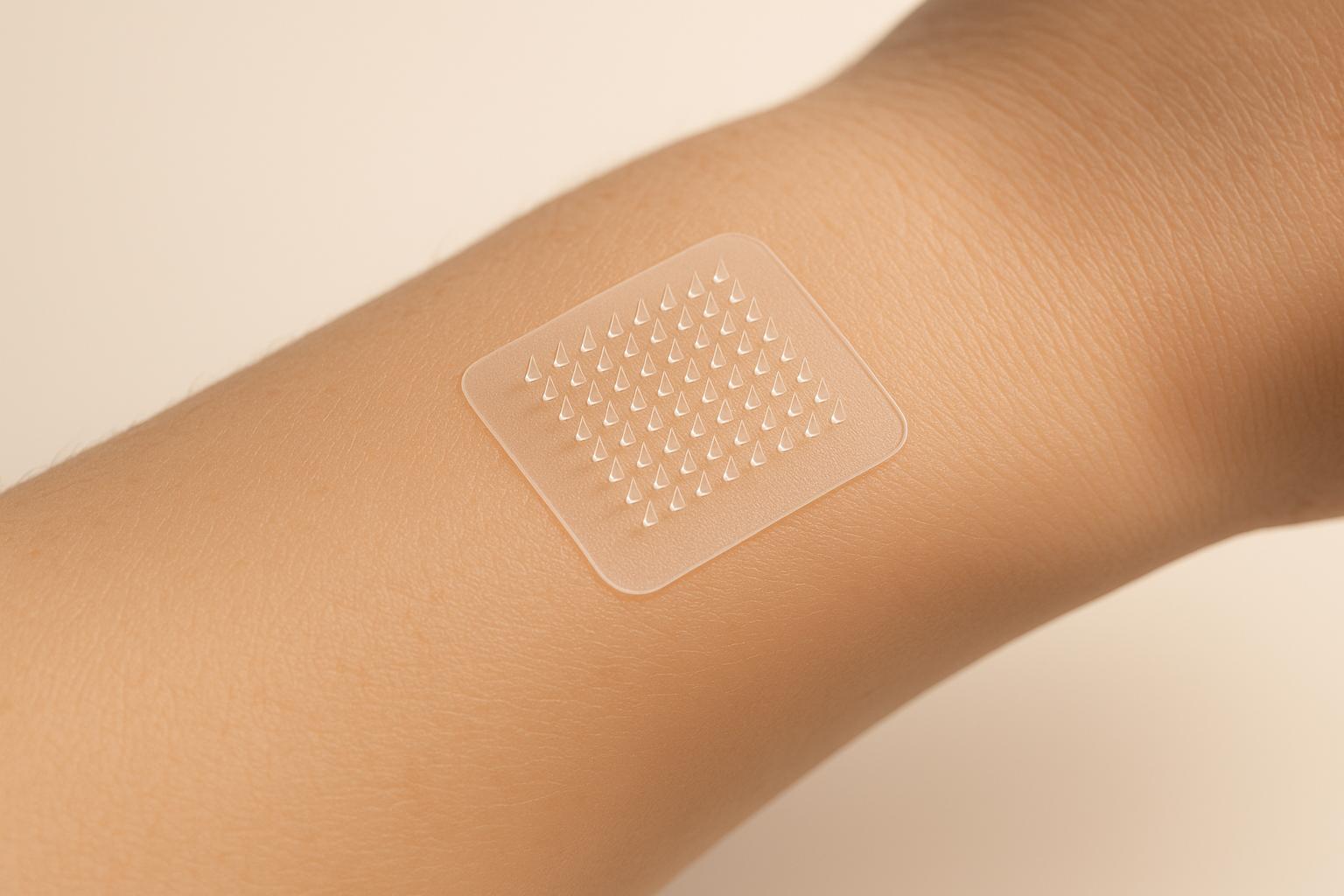
Polymeric microneedles offer a new way to deliver metformin through the skin, bypassing the digestive system to reduce side effects and improve absorption. These tiny, painless needles create microchannels in the skin, allowing controlled drug delivery without reaching pain receptors. Here's what you need to know:
- Why It Matters: Oral metformin often causes stomach discomfort and has limited bioavailability (50–60%). Microneedles avoid these issues by delivering the drug directly into the skin.
- How They Work: Made from biocompatible polymers, microneedles dissolve, swell, or use reservoirs to release metformin steadily or on demand.
- Key Benefits: Better absorption, fewer side effects, and easy self-application make them ideal for chronic conditions like diabetes.
Researchers are also exploring advanced systems, including near-infrared (NIR)-responsive microneedles for precise, on-demand dosing. This technology could transform diabetes management by offering more effective, patient-friendly treatments.
How do microneedles deliver drugs? | The latest in drug administration technology
How Polymeric Microneedles Penetrate the Skin
Polymeric microneedles are designed to pierce the skin's outer barrier with minimal discomfort. By creating tiny, temporary channels, they enable drugs to be delivered through the skin without reaching deeper layers where nerve endings are located.
How Microneedles Enter the Skin
These microneedles work by forming short-lived pathways through the stratum corneum, the outermost layer of skin, which is only about 10–20 μm thick. Their design ensures they penetrate this barrier but stop before reaching the dermis, where nerve endings reside (approximately 1,000 μm deep). This feature significantly reduces pain. The process of insertion involves three stages: the skin first deforms, then the microneedle penetrates, and finally, the skin relaxes as the needle is withdrawn. A force of just 0.03 mN at the needle tip is enough to overcome the skin's resistance.
The dimensions of the microneedles are crucial for a pain-free experience. Most microneedles are 150 to 1,500 μm long, 50 to 250 μm wide, with tip diameters ranging from 1 to 25 μm. Studies show that increasing the needle length from 480 μm to 1,450 μm raises pain perception from 5% to 37%. Additionally, the geometry of the needle - such as the tip shape, diameter, and aspect ratio - affects how much force is needed for penetration. For instance, pyramid-shaped microneedles are often more durable due to their larger cross-sectional area. Research also indicates that silicon microneedles around 150 μm tall can boost drug penetration rates by up to 10,000 times compared to topical applications.
Skin characteristics like stiffness, thickness, and elasticity also impact how effectively microneedles penetrate. For example, the skin on the forehead is generally thicker and stiffer compared to the forearm. Once microneedles create microchannels, these tiny pores naturally begin to close. Their diameter shrinks by about 25% within the first 30 minutes and nearly seals completely after about six hours.
Now, let's examine the materials that make these microneedles both effective and safe.
Materials Used in Polymeric Microneedles
The choice of materials for polymeric microneedles is critical. These materials must provide enough strength to pierce the skin while also supporting controlled drug release.
PLGA (Poly(lactic-co-glycolic acid)) is a popular choice due to its strong mechanical properties. With an elasticity modulus of 1–3 GPa and tensile strength between 50–100 MPa, PLGA microneedles can easily penetrate the skin. They degrade slowly, allowing for sustained drug release over days or even months.
PVP (Polyvinylpyrrolidone) is a fast-dissolving material, often dissolving within a minute of insertion. This makes it ideal for quick drug delivery. However, its mechanical strength is relatively low, so it is often blended with other materials. For example, mixing PVP with 1% methacrylic acid (MAA) can double its strength, while a 25% MAA blend can make it up to four times stronger.
PVA (Polyvinyl alcohol) is frequently combined with PVP to enhance hardness and overall performance. Unlike PVP, cross-linked PVA microneedles do not dissolve in the skin, which allows them to be removed intact after delivering the drug.
Hyaluronic Acid (HA) is known for its excellent compatibility with the body. It dissolves within about 10 minutes after insertion. Although its elasticity modulus is lower (roughly 0.1–0.5 GPa), HA is still effective in penetrating the skin and can be blended with other polymers for added strength.
The mechanical properties of these materials are carefully balanced to ensure successful skin penetration while minimizing the risk of damage. For instance, harder materials like PEEK (polyether ether ketone) offer greater strength and abrasion resistance but may increase the likelihood of irritation. On the other hand, water-soluble polymers tend to have lower mechanical strength, which is why manufacturers often blend materials and adjust factors like cross-linking density, molecular weight, and processing temperatures to achieve the desired performance. This careful material selection ensures safe and effective drug delivery, including for medications like metformin.
Drug Loading and Release Methods
After creating microneedles, the next critical step is incorporating metformin and managing its release. The method used for loading the drug not only determines its concentration within the microneedle matrix but also influences the release profile and overall therapeutic impact.
Incorporating Metformin into Microneedles
There are several techniques for embedding metformin into polymeric microneedles, each tailored to achieve specific release profiles and clinical goals:
- Direct Mixing: This involves blending metformin directly with the polymer during fabrication. For example, combining metformin powder with a photo-curable resin creates a porous structure that allows controlled drug release.
- Coating Methods: These precisely apply metformin to the tips of the microneedles, ensuring sustained delivery as the needles remain embedded in the skin.
- Nanoparticle Carriers: This approach uses hollow mesoporous silica nanoparticles to load metformin. These particles are then coated with polydopamine and lauric acid, forming carriers responsive to near-infrared light, which are incorporated into the microneedles.
- Reservoir-Based Systems: These pair hydrogel-forming microneedles with separate drug reservoirs. A lyophilized blend of metformin HCl, gelatin, mannitol, and water achieves over 90% drug recovery, ensuring both stability and efficiency.
For the best stability and delivery performance, the drug concentration in the microneedle matrix should stay below 50%, with the maximum drug-to-microneedle component ratio typically being 1:1.
These methods pave the way for a variety of release mechanisms, as discussed below.
Dissolving and Swelling Microneedle Systems
When applied, dissolving microneedles break down in the interstitial fluid, while swelling microneedles absorb fluid, expanding to create diffusion channels. The polymer's properties, such as hydrophilicity and degradation rate, play a significant role in the dissolution rate.
For instance, hydrogel-forming microneedles made from a 20% w/w poly(methylvinylether-co-maleic acid) blend crosslinked with 7.5% w/w poly(ethylene glycol) store metformin in a separate lyophilized drug reservoir. This system activates upon contact with body fluids, enabling controlled release.
The physical state of metformin also matters. Amorphous forms generally release the drug more efficiently than crystalline forms due to better dissolution properties. Additionally, the microneedle design significantly affects insertion efficiency. Cone-shaped microneedles, for example, achieve an insertion efficiency of about 98%, outperforming designs like cone-cylinder (39%), rectangular pyramid (50%), or hexagonal pyramid (76%). For effective skin penetration, a tip diameter of 10–15 μm and a tip-to-tip spacing of over 500 μm are recommended.
Delivery efficiency can be further improved through formulation techniques. Using co-solvency methods and optimized solvent systems, such as ethanol/water mixtures, enhances the mechanical strength and insertion performance of microneedles, facilitating quicker drug delivery. External triggers, like near-infrared light, can also be incorporated to enable on-demand drug release, offering precise dose control.
These advanced strategies for drug loading and release highlight the potential of polymeric microneedles in metformin delivery.
sbb-itb-cad6158
Advanced Controlled and Responsive Release Technologies
Researchers have gone beyond the basic dissolving and swelling mechanisms to create advanced systems that can precisely control how metformin is released. These innovations allow healthcare providers and patients to tailor drug delivery to individual needs. Let’s dive into these cutting-edge methods, starting with NIR-responsive systems.
Near-Infrared (NIR) Responsive Systems
NIR-responsive microneedles act like a remote-controlled drug delivery system. They use materials that convert near-infrared light into heat, which then melts or breaks down the microneedle matrix to release metformin. For example, at Zhejiang Sci-Tech University, researchers developed microneedles using Prussian blue nanoparticles as photothermal agents. When exposed to NIR light, these nanoparticles generate heat, melting polycaprolactone arrowheads loaded with metformin and releasing the drug on demand.
Another approach involves hollow silica nanoparticles coated with polydopamine and lauric acid. Here, polydopamine absorbs NIR light and generates heat, which melts the lauric acid coating, triggering the release of metformin. The timing and dosage can be fine-tuned by adjusting the exposure time and intensity of the NIR light. In lab studies, this method achieved a metformin bioavailability of 95.8% ± 2.7%, almost matching the effectiveness of subcutaneous injections.
Weijiang Yu et al. noted, "They allowed on-demand control of timing and dose of the drug released. This suggests that the developed NIR-triggered and separable MNs are a promising transdermal drug delivery system that enables the patient or physician to adjust therapy precisely in an active manner, thus improving treatment efficiency and reducing side-effects."
Next, let’s explore back-layer reservoir designs, another promising option for sustained drug release.
Back-Layer Reservoir Designs
Back-layer reservoir systems take a different approach by separating drug storage from the delivery mechanism. Instead of loading metformin directly into the microneedles, these systems store the drug in a reservoir attached to the microneedle’s backing layer. The microneedles create pathways to connect the reservoir to the skin’s dermal circulation, allowing the drug to be delivered over an extended period.
One example is a two-layer system that uses swellable microneedles and a lyophilized metformin reservoir. When applied to the skin, the needle tips absorb skin fluid, swelling to form continuous channels that allow the metformin to flow from the reservoir to the skin’s microcirculation. This setup can sustain drug release for up to 24 hours.
This method offers several advantages. The extended release reduces the need for frequent applications, improving patient compliance and helping maintain stable blood sugar levels throughout the day.
Comparison of Release Methods
| Release Method | Release Mechanism | Key Advantages | Primary Limitations | Ideal For |
|---|---|---|---|---|
| Dissolving | Microneedle dissolves in skin fluid | Simple, one-step application; complete drug release | Fixed release rate; cannot be stopped once applied | Daily dosing; convenience-focused patients |
| Swelling | Needles absorb skin fluid to create diffusion channels | Controlled release; potential for fluid sampling | Slower initial release; depends on skin hydration | Extended therapy; monitoring combinations |
| NIR-Responsive | NIR light generates heat to release the drug | Precise, on-demand dosing; adjustable timing | Requires an external NIR device; more complex setup | Personalized therapy; variable dosing needs |
| Reservoir-Based | Drug stored in a reservoir, delivered via microneedle channels | High drug capacity; prolonged release (24+ hours) | More complex manufacturing; larger patch size | Long-term therapy; fewer daily applications |
Each method has its strengths and is suited to different patient needs. NIR-responsive systems shine in situations where precise, on-demand dosing is critical, while reservoir-based designs are perfect for long-term, consistent drug delivery with minimal effort.
These advanced technologies provide new ways to deliver metformin through the skin, giving patients and healthcare providers greater control over diabetes management.
Clinical Potential and Future Directions
Polymeric microneedles for metformin delivery are more than just a novel technology - they represent a potential game-changer in diabetes management. With promising early results and ongoing research, these systems could redefine how patients receive treatment. Let’s break down their impact on patient adherence, key research findings, and what lies ahead.
Improved Patient Adherence and Safety
One of the toughest hurdles in diabetes care is ensuring patients stick to their medication schedules. Oral metformin often causes gastrointestinal side effects, leading many to skip doses. Microneedles bypass the digestive tract entirely, minimizing these side effects. They also offer a steady, controlled dosing schedule, making it easier for patients to stay consistent with their treatment.
This controlled release mechanism not only simplifies the regimen but also ensures more precise dosing and stable therapeutic levels. Early studies back up these benefits, showing promise for better adherence and outcomes.
Key Research Findings: Preclinical and Early Clinical Studies
Research into microneedles for metformin delivery has already shown impressive results. For instance, a 2018 study by Migdadi et al. tested hydrogel-forming microneedles and saw a significant boost in metformin delivery. Using neonatal porcine skin, the microneedle system delivered 9.71 ± 2.22 mg at 6 hours and 28.15 ± 2.37 mg at 24 hours. In contrast, setups with only drug reservoirs delivered just 0.34 ± 0.39 mg at 6 hours and 0.39 ± 0.39 mg at 24 hours.
Animal studies have been equally promising. In rats, metformin levels in plasma reached 0.62 ± 0.51 μg/mL within an hour of using microneedles, with levels peaking at 3.77 ± 2.09 μg/mL after 24 hours. The transdermal bioavailability was estimated at around 30%.
Other breakthroughs include Liu et al.’s rapidly separable microneedles, which provided a longer-lasting hypoglycemic effect in diabetic rats compared to traditional injections. Abbasi et al. combined microneedles with iontophoresis to deliver metformin directly to subcutaneous white adipose tissue in obese mice, leading to reduced fat gain, increased energy expenditure, and improved metabolism through the browning of white fat tissue.
What’s Next: Smart Drug Delivery Systems
Building on these findings, researchers are working on microneedle systems that combine real-time monitoring with responsive drug delivery. Imagine a microneedle patch that not only tracks glucose levels but also adjusts the release of metformin accordingly - a closed-loop system for managing diabetes.
The demand for these innovative systems is growing rapidly, with 537 million adults worldwide living with diabetes . The market for microneedle technologies is expected to expand significantly as a result.
"Smart microneedles are poised to play a significant role in advancing personalized and noninvasive medical treatments."
Future microneedle systems may include stimuli-responsive designs that release drugs based on changes in glucose levels, pH, temperature, or specific enzymes. For example, a patch could automatically release more metformin when blood sugar levels rise, offering a tailored approach to therapy.
Nanotechnology is also opening new doors. Researchers are exploring combinations with liposomes, dendrimers, and metallic nanocarriers to create even more advanced delivery systems. Bioresponsive polymers, which adapt their properties to physiological changes, could enable microneedles that adjust drug release rates in real time based on blood sugar levels.
As materials science, nanotechnology, and digital health tools continue to evolve, these smart drug delivery systems could transform diabetes care, making treatment more personalized, efficient, and patient-friendly.
Conclusion
Polymeric microneedles are changing the game when it comes to metformin delivery, offering a smarter way to tackle some of the biggest challenges in diabetes management. By bypassing the digestive system, these devices sidestep common side effects that often lead to poor patient adherence.
What makes microneedles stand out is their ability to improve metformin's pharmacokinetics by avoiding first-pass metabolism. They create tiny microchannels in the skin, which dramatically boost absorption compared to traditional transdermal methods. This precise delivery into the epidermis and dermis ensures better drug uptake and effectiveness.
These systems are not only painless and easy to self-administer but also cost-effective, making treatment more accessible. As Muhammad Bilal, PhD, puts it:
"MNs are becoming a smart approach with time from the conventional transdermal approach. These are preferred over hypodermal needles because of the painless nature of MNs."
Another key advantage is their controlled release mechanism, which provides steady and sustained drug delivery. This helps maintain consistent therapeutic levels, reducing dosing fluctuations and improving both patient compliance and treatment stability.
In short, polymeric microneedles offer the perfect blend of convenience, precision, and patient comfort. With millions of people worldwide living with diabetes, this approach has the potential to transform treatment outcomes and significantly enhance quality of life. Beyond that, it opens the door to more advanced, responsive drug delivery systems in the future.
FAQs
How do polymeric microneedles enhance the delivery and effectiveness of metformin compared to taking it orally?
Polymeric microneedles offer a game-changing approach to delivering metformin by sidestepping the digestive system entirely. This means they avoid the first-pass metabolism - a process that often reduces the effectiveness of orally administered drugs - and allow the medication to be absorbed directly into the bloodstream. While oral metformin typically has a bioavailability of about 50-60%, microneedles provide a more reliable and consistent therapeutic effect by delivering the drug through the skin.
Another advantage of this method is its ability to release the drug gradually over time. This sustained release can help minimize the gastrointestinal side effects that are commonly associated with oral metformin. By piercing the skin's outer layer, these microneedles ensure precise and efficient drug delivery, making them a more targeted and potentially effective alternative to traditional pills.
What are the benefits of using near-infrared (NIR) microneedles for managing diabetes?
Near-infrared (NIR) microneedles offer a gentle and pain-free method to manage diabetes by delivering insulin with accuracy and control. These microneedles are activated by NIR light, enabling on-demand insulin release that minimizes the risk of hypoglycemia and improves treatment effectiveness.
When exposed to NIR light, these microneedles quickly heat up, allowing for precise and targeted drug delivery. This approach not only enhances the convenience of treatment but also supports customized and adaptable therapy, paving the way for better diabetes management while increasing patient comfort and adherence.
What makes the materials in polymeric microneedles effective and safe for delivering Metformin?
Polymeric microneedles are crafted using skin-friendly and biodegradable materials like hyaluronic acid (HA), polyvinylpyrrolidone (PVP), and poly-L-glutamic acid (γ-PGA). These substances dissolve harmlessly within the skin, ensuring smooth drug delivery while reducing the chances of toxicity or lingering tissue damage.
In addition, polymers approved by the FDA, such as poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and chitosan, are commonly used. These materials not only allow for controlled drug release but also naturally break down in the body, making them a safe and effective option for delivering Metformin while keeping patient safety at the forefront.
.png)



